💨 ONZETRA® Xsail® (Breath-Powered Sumatriptan)
A Needle-Free, Fast-Acting Migraine & CH Treatment — and a Product to Watch in Australia
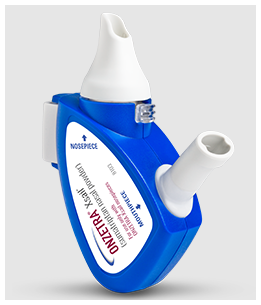
ONZETRA® Xsail® is a unique medication used overseas (mainly in the USA) for the fast delivery of sumatriptan. Instead of being a liquid nasal spray or an injection, it uses a breath-powered device to deliver a dry powder form of sumatriptan deep into the nasal passages where it absorbs quickly.
It is not currently available in Australia, but many patients and clinicians consider it an important product to watch, especially now that sumatriptan injections and nasal sprays have been discontinued locally.
Email Currax about ONZETRA in AustraliaWhat Makes ONZETRA Different?
1. Breath-Powered Delivery
You place the device in one nostril, blow gently into the mouthpiece, and your breath pushes the powder directly into the high-absorption area at the back of the nose.
No dripping, no swallowing, no bad aftertaste.
2. Fast Relief
Because the powder is absorbed through the nasal blood vessels (instead of the stomach), pain relief can begin as early as 15–30 minutes for many users.
3. Needle-Free Option for Triptan Users
ONZETRA offers a way to get the benefits of sumatriptan without injections, which many people dislike or struggle to access.
What Does the Research Say?
Clinical studies show:
🔹 Relief starts faster than with oral sumatriptan
🔹 Lower systemic exposure → fewer whole-body side effects
🔹 Designed to avoid the common issues with ordinary nasal sprays (drip, slow absorption, bad taste)
Who Is It For?
ONZETRA is approved in the USA for:
-
Adults with acute migraine, with or without aura
It is not approved for cluster headaches but only because it wasnt tested in Cluster Headache trials. although the delivery technology may be of interest to the CH community in the future.
Why This Product Matters in Australia
With the recent discontinuation of sumatriptan injections and nasal sprays in Australia, many patients are left with fewer fast-acting options.
ONZETRA is one of the most promising alternatives overseas because:
-
It delivers triptan medication quickly,
-
without needles,
-
without swallowing tablets,
-
and without the slow onset of traditional sprays.
If it becomes available in Australia, it could fill an important gap in treatment options.
Important Note
ONZETRA® Xsail® is NOT currently sold or registered in Australia.
This information is provided for educational purposes only.
Patients should not attempt to import prescription medications without consulting a doctor and reviewing TGA regulations.
But given current changes in Australian triptan availability, ONZETRA is absolutely a product to watch.
GEtting It in Australia
Getting a medication like ONZETRA® Xsail® approved in Australia isn’t simple, but it is possible — and there are several realistic pathways that patients can help influence.
Here’s the clearest breakdown of how a drug gets approved, what needs to happen, and what you (and the CH/migraine community) can do to push it forward.
1. A company must apply to the TGA (Therapeutic Goods Administration)
This is the single biggest barrier.
ONZETRA cannot be approved unless the manufacturer — Currax Pharmaceuticals (formerly Avanir/Optinose affiliations) — submits:
-
A formal application
-
Clinical trial data
-
Safety data
-
Manufacturing quality documentation
This is the #1 reason many medications never reach Australia:
companies don’t think the Aussie market is big enough to bother.
2. What would motivate the company to apply?
Two things:
1. Market demand
If enough individuals, clinics, neurologists, and advocacy groups express need, companies may reconsider.
Pharma companies do respond to coordinated, high-volume patient interest.
2. Financial viability
Australia’s market is small, and ONZETRA is a niche product.
That means the manufacturer needs to believe:
-
Doctors will prescribe it
-
Pharmacies will stock it
-
The PBS may subsidise it
This only happens if the community makes the case loudly and consistently.
Get neurologists involved
If clinicians request it, this substantially increases company interest.
Ask neurologists to send letters to Currax or to the TGA directly saying:
“There is a clinical gap in fast triptan delivery systems in Australia, especially with the discontinuation of sumatriptan injections. ONZETRA fills an unmet need.”
Doctors carry weight.
Organise a patient petition
A petition with:
-
migraine patients
-
cluster headache patients
-
neurologists
-
headache clinics
-
pain specialists
…creates public pressure and makes pharma companies reconsider.
Engage an advocacy organisation
These groups can pressure pharma in ways individuals can’t:
-
Migraine Australia
-
Headache Australia
-
Pain Australia
-
RARE Voices Australia
If they adopt the cause → much higher visibility.
Realistic timeline (if the company decides to apply)
If Currax files the TGA application:
-
Evaluation: 1–2 years
-
Approval: another 6–12 months
-
PBS listing: 1–3 years after approval (optional but helpful)
With coordinated pressure, it can be done — but it won’t be fast.
What you can do right now (and it actually helps)
Write to Currax Pharmaceuticals (the manufacturer)
Tell them:
There is a large unmet need in Australia
Sumatriptan injections & sprays were discontinued
Patients urgently need fast-acting alternatives
ONZETRA is uniquely positioned to fill that gap
You personally, and your patient community, would use it
A collective push does influence companies.
- Onzetra® Product website
- Contact Currax Phamaceuticals
Here is a letter you can copy and use if the email button above doesn't work:
Subject: Request for Australian Availability of ONZETRA® Xsail® (Breath-Powered Sumatriptan)
To the Currax Pharmaceuticals Team,
I am writing as an Australian patient advocate on behalf of a large and rapidly growing community of migraine and cluster headache patients. We are reaching out to express strong interest in the availability of ONZETRA® Xsail® in Australia and to ask whether Currax Pharmaceuticals has considered seeking registration with the Therapeutic Goods Administration (TGA).
Recently, Australia experienced a significant loss in acute headache treatments:
both the sumatriptan subcutaneous injection and the sumatriptan nasal spray were discontinued by Aspen Pharma, leaving patients without the fast-acting options we rely on.
This change has created a major clinical and patient-safety gap, especially for:
Migraine patients who require rapid-onset treatment
Cluster headache patients who depend on fast abortives
Individuals who cannot tolerate oral triptans
Patients who need portable, needle-free options
This vacuum in the Australian market has left thousands of people struggling to manage acute attacks safely and effectively.
Because of this, there is now a clear unmet need for an intranasal, fast-acting sumatriptan delivery system in Australia.
ONZETRA® Is Uniquely Positioned to Fill This Gap
ONZETRA’s breath-powered dry-powder delivery system offers several advantages that make it especially well-suited for our population:
Faster onset than oral sumatriptan
Needle-free, easy to use
Highly efficient nasal absorption
Reduced systemic exposure
Ideal for patients unable to swallow or retain oral medications during attacks
As more Australian patients learn about ONZETRA, interest is rapidly increasing. Many have expressed willingness to pursue Special Access Scheme (SAS-B) prescriptions — a far more difficult and expensive pathway — simply because no local alternatives remain.
The Australian Patient Community Would Strongly Support ONZETRA
Through patient groups, support communities, and clinician networks, it is clear that ONZETRA would be widely used here if available. Clinicians who previously prescribed injectable sumatriptan are seeking replacement options and have begun recommending intranasal treatments — but none currently match what ONZETRA offers.
Request for Information
We respectfully ask:
Has Currax considered applying for TGA approval for ONZETRA® Xsail® in Australia?
Is Currax open to exploring the possibility of Australian distribution now that competing products have been discontinued?
What steps can patient groups take to help demonstrate the level of demand required for a market assessment?
Bringing ONZETRA to Australia would not only support hundreds of thousands of patients, but also fill a vital gap in fast-acting triptan treatment at a time when we have been left with very limited options.
Thank you for taking the time to consider this request.
We would be grateful for any guidance or next steps you can provide.
Sincerely,
[Your Name]
[City, Australia]
[Optional: Patient organisation or group]
[Email / Contact]